what to do when coal is fusing in tuiyers
An increasing supply of depression-cost higher-chlorine coal is prompting many U.S. generators to give the fuel serious consideration in spite of its reputation for causing corrosion. Though corrosion can be a consideration, it'south not ever caused simply past the fuel switch. Understanding the various chemical interactions, likewise equally operational and emissions pros and cons, can increase the odds of success with this blazon of fuel.
With the installation of flue gas desulfurization (FGD) systems at most larger U.S. coal-fired power plants, operators are increasingly using high-sulfur Illinois Basin coal from Illinois, western Kentucky, and Indiana (the ILB). To meet this growing demand, new mines are being built in the ILB. The largest and nearly efficient of these new mines produce coal with over 0.2% chlorine; some even exceed 0.3% chlorine. Opinion in the ability industry is divided almost whether this higher-chlorine coal tin can be burned without meaning increases in banality fireside corrosion. Chloride aggregating in FGD systems is likewise a concern with these dress-down.
In addition to corrosion considerations, coals with college chlorine content have pros and cons when it comes to emissions. They can increase the removal of mercury in wet FGD systems, as chlorine helps to oxidize the mercury into a soluble grade. For compliance with the Environmental Protection Bureau'south (EPA'due south) Mercury and Air Toxics Standards (MATS), some plants will benefit from this effect. At the same time, hydrogen chloride gas is an acrid gas regulated nether the MATS rules, and higher chlorine could increase its product in the banality.
ILB production has grown to xiv% of U.Southward. coal product, from 8% in 2008 (Figure 1). Higher-chlorine coal production in 2013 was nigh 17 million tons, and 28 one thousand thousand tons/year of new higher-chlorine coal product is expected to exist added over the next ii to three years. Coal from these mines is likely to be ane of the lowest-cost fuels available to power plants in the eastern U.South. Higher-chlorine coal from the ILB has also entered the world steam coal market via the Port of New Orleans, with exports to Europe, especially England.
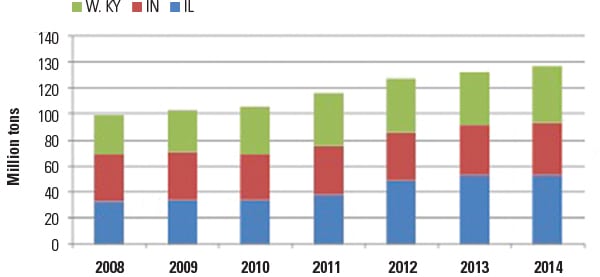 |
| 1. Illinois Basin coal product, historical and estimated. 2014 estimates are based on weekly coal product through Nov. 29, 2014. Source: Free energy Information Administration |
Some generators have excluded the use of coal with more 0.15% chlorine or, sometimes, 0.2% chlorine. This article considers whether coal with higher chlorine levels can be successfully used and whether boiler corrosion might occur even with lower-chlorine ILB coal. Both the literature on the effect of chlorine and experience at a number of plants are discussed.
What'south Known and Unknown Almost Chlorine and Corrosion
The literature on the effects of chlorine on banality tube corrosion is somewhat contradictory. Some sources assert that there is a clear correlation betwixt the chlorine content of coal and the extent of corrosion, with some asserting that coal with over 0.two% chlorine should exist avoided. Some of these same researchers suggest a linear human relationship between chlorine and corrosion, in which case at that place would be no meaningful "safe" threshold value.
What appears to be missing is any big-scale (in the 500-MW range) longer-term test in which high- and low-chlorine coals have been compared. Instead, pilot scale tests and considerable computer boiler simulations accept been done, along with some express commercial-calibration investigation.
Several conclusions emerge from this literature:
■ The physical and chemical phenomena in the boiler that determine chlorine-induced corrosion are extremely complicated. Chlorine interacts with other coal constituents, including alkali metals, atomic number 26 (Fe), and sulfur (S). The compounds that grade are strongly influenced by the oxygen balance in the banality atmosphere and the presence of unburned carbon.
■ Therefore, as boiler operating conditions change from reducing to oxidizing, the slag chemistry and corrosion mechanisms shift in important ways. The most severe corrosion appears to occur when shifts in boiler oxygen balance occur.
■ Command of the corrosive effects of high-chlorine coal and loftier-FeS coal are closely related. Chlorine-induced corrosion becomes significant when deposits of Fe- and S-containing slag form.
■ Some banality operators have, over a long flow, reported use of higher-chlorine coal with acceptable boiler corrosion.
■ Operating practices that provide skilful flame control, slag direction, and avoidance of reducing zones along the boiler waterwall allow successful utilize of higher-chlorine (and high-sulfur, high-slagging) coal.
Why Chlorine Matters
Chlorine is present in substantial amounts in almost all Appalachian and Illinois Basin coal production, every bit illustrated in Effigy two, with nigh production in the range of 0.one% to 0.2% chlorine. Based on the 1999 data used for Figure ii, the tonnage-weighted boilerplate chlorine content of the coal from Illinois mines was 0.21% and for western Kentucky mines, 0.12%, while the widely used Pittsburgh seam coals have almost 0.1% chlorine.
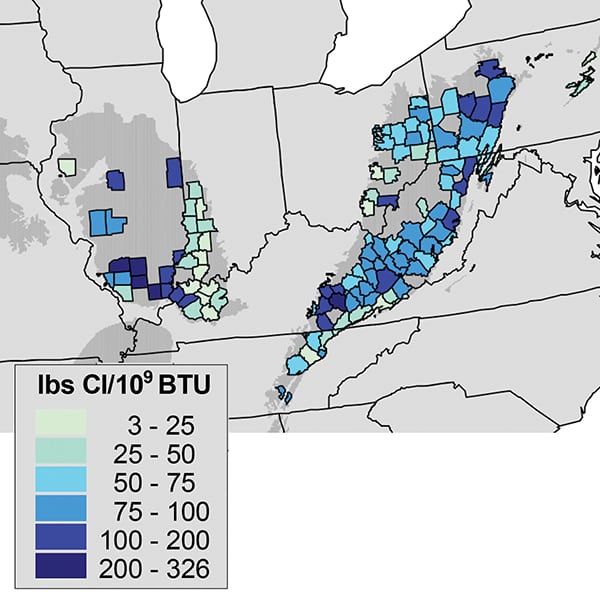 |
| 2. Variable chlorine content. This map shows average chlorine content of coal delivered to U.South. power plants during 1999 by canton of origin (calculated using selected EPA Information Collection Request data). Source: Quick, Jeffrey, et al., "Optimizing Technology to Reduce Mercury and Acrid Gas Emissions from Electric Power Plants," 2005 |
Chlorine reacts with alkali metal elements in the ash—calcium (Ca), magnesium (Mg), sodium (Na), and potassium (K). As discussed below, a complex series of reactions tin can occur between chlorine and the atomic number 26 and sulfur in banality wall slag deposits. Therefore, the effects of chlorine in the banality depend strongly on the overall ash content, slag forming trend, and Atomic number 26 and Southward content. High-Fe and Southward coal tends to grade slag. This slag interacts with chlorine and may accelerate corrosion.
Tabular array one shows the chemistry of several representative coals. The Illinois Basin and Northern Appalachian (NAPP) coals have lower ash fusion temperature and college Fe and Southward content than Central Appalachian (CAPP) coal or Powder River Basin (PRB) coal.
 |
| Tabular array ane. Quality characteristics of case coal types. Sources: (1), (3) Foster Wheeler, 2009; (2) White Oak Resources LLC.; (four) confidential customer |
The lower ash fusion temperatures of the ILB, PRB and NAPP coals compared to CAPP coal tends to exist associated with greater germination of banality slag. The interaction of chlorine with boiler slag is central to agreement the effects of chlorine in coal. In other words, it is not only the chlorine that matters.
Chlorine and Furnace Tube Corrosion
In the absence of slag deposits, chlorine in the grade of gaseous hydrochloric acrid (HCl) causes just small banality tube corrosion. Where boiler slag accumulates, particularly Fe-containing slag, and chlorine is present, accelerated corrosion may occur.
Slag forms in the banality when molten ash contacts the banality surfaces (the waterwall and superheater sections, where temperatures can be higher up the ash melting point). Slag affects heat transfer in the furnace and must therefore be controlled. Slag is controlled through some combination of fuel selection and operating practices, including apply of sootblowers, factory and burner settings, and output level (such every bit cycling to cool and de-slag superheater surfaces).
Corrosion of boiler tubes mostly occurs underneath the slag layer. Underneath the slag, liquid layers can form depression-melting-point compounds that segregate from the coal ash. There are many reaction mechanisms, including germination of iron sulfide and fe chlorides. These compounds may exist volatile or flake away from the boiler tubes, exposing fresh metal surfaces to farther corrosion. Therefore, there is a strong human relationship between the extent of slagging and the extent of corrosion.
Chlorine and sulfur are frequently present together in the coal, and their effects are connected. Corrosion tin can consequence from the activity of sulfur as well as chlorine, especially under reducing conditions.
Corrosion from high-Atomic number 26 coal is more than severe when reducing weather occur in the boiler. The operation of depression-NOx burner (LNB) and overfire air (OFA) systems intentionally lowers the oxygen level in the lower portions of the banality, where primary combustion takes place. Depending on the operation of coal mills, piping, and burners, molten ash particles containing FeS2 (pyrite) or FeS may be deposited in the slag (sometimes called "impingement" of ash). As noted below, in reducing conditions, these deposits are corrosive.
Figure iii illustrates in concept how large, depression-melting corrosive iron sulfides reach the waterwall. Lowering main airflow and finer grinding will encourage a shift from aggregating of wall slag to formation of dry out fly ash.
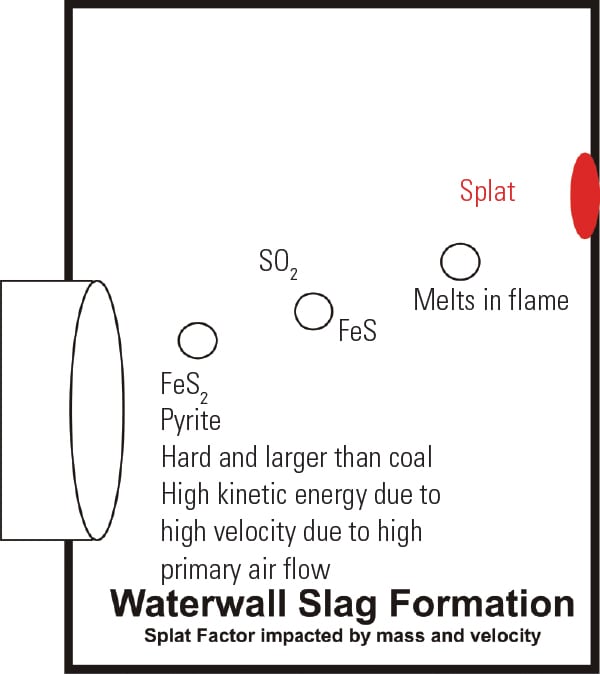 |
| 3. Waterwall slag germination. This diagram shows a coarse pyrite particle that is decomposed in the flame (from FeS 2 to FeS), melts, and has sufficient momentum to "splat" confronting the waterwall, forming slag. This particularly occurs when grinding is poor and the coal pipe/burner velocities are high. Source: Coal Combustion Inc. |
Chlorine can combine with iron and sulfur in slag deposits in areas of the banality where reducing atmospheric condition occur to grade compounds that can corrode the wall tubes. Every bit the coal burns, the chlorine will initially form HCl. That HCl reacts with the alkali metals Na and K to form NaCl and KCl, which have a low melting signal. If these compounds are present in the slag, they can class a molten corrosive layer on the boiler tubes.
If, equally is usually the case, sulfur is too present in the flue gas, And so3 replaces the chlorine and sulfate deposits form. Sulfate deposits accept higher melting points than chlorides and are less corrosive. Therefore, the residuum between chlorine and sulfur is important. Still, every bit discussed below, when transitions occur between reducing and oxidizing weather in the boiler, every bit may occur due to changes in load, the corrosive effects of both sulfur and chlorine may increase profoundly.
The chemic species present in the coal ash are very sensitive to the flue gas oxygen/carbon monoxide concentrations. Most areas of the boiler accept enough oxygen to maintain "oxidizing" conditions. Where oxygen is scarce in relation to the carbon present, "reducing" weather occur. Oxidizing conditions will be establish in the upper portions of the boiler during most levels of operation and throughout the banality at lower output levels. At loftier output levels, and with LNB OFA systems, the lower portion of the banality may have areas of reducing conditions. Therefore, as a boiler is cycled betwixt low and high output, some portions of the boiler will shift betwixt reducing and oxidizing conditions.
Iron in slag deposits reacts with the flue gas. If the flue gas is reducing, the reduced forms of Iron—Fe (II) or metallic iron—predominate. If conditions are oxidizing, and so oxidized iron—Fe (III)—will predominate. The reduced atomic number 26 has a lower melting signal and is more likely to form a molten deposit of FeS on the boiler tube. The sulfur attacks the tube metal. Chlorine may be present in the course of brine chlorides, which are somewhat corrosive, depending strongly on temperature.
If the boiler atmosphere shifts from reducing to oxidizing weather condition, the brine chloride compounds in the slag convert to brine sulfates (stable) and release corrosive HCl or Cl2 gases.
The contrary situation, a shift from oxidizing to reducing conditions can besides occur. Stable slag deposits formed in oxidizing atmosphere containing sulfates tin release corrosive sulfide if weather condition shift to reducing atmosphere.
Based on substantial feel with coals with a range of chlorine content, from <0.i% to over 0.3% Cl, there is not a direct human relationship between chlorine content and the extent of banality corrosion.
This event, as noted higher up, is consistent with the interactions among chlorine, sulfur, and boiler operating conditions. There is no doubtfulness that ILB and NAPP (higher-sulfur, low-fusion) coals can produce boiler corrosion, merely several mechanisms are at work.
Probably the best explanation for the lack of clear correlation betwixt chlorine content and the extent of corrosion is that other factors—including sulfur, brine content, total ash loading, and banality atmosphere control—are as great or greater influences on observed corrosion. The implication is that some boilers might experience serious corrosion while using a coal with <0.2% Cl and coal with >0.3% Cl might be adequate at other units. There are many years of experience with Illinois dress-down that have chlorine content at or above 0.2%.
The implications of these concrete and chemical factors are that:
■ Chlorine can contribute to corrosion of banality tubes.
■ The extent of corrosion depends on the amount of slag formation.
■ Sulfur in the coal tends to reduce the corroding upshot of chlorine.
■ Corrosion will exist more astringent in areas of the boiler with reducing flue gas conditions.
These problems are likely to become credible when boilers formerly fueled with CAPP coal (low sulfur, high fusion) brainstorm to use ILB (or NAPP) coal, with its higher slagging tendency. This will be especially true, as discussed below, where LNB OFA control systems produce areas of reducing temper in the banality.
Slag Control
The extent of corrosion from chlorine (and other coal constituents) depends strongly on the presence of long-lasting slag deposits.
Slag control (typically using sootblowers) is a regular role of banality operation with high-sulfur low-fusion coals. A thorough survey of sootblower performance and refurbishment, where necessary, is essential for successful employ of ILB coal, specially if chlorine is a concern. A best-practice standard is visual slag checks three times per shift and repair of malfunctioning sootblowers within half-dozen hours, before serious slag accumulation tin occur.
Given a particular coal, in cases where more severe slagging is experienced, boiler operators may modify some banality parts (such as changing superheater tube layout) or reducing allowable maximum load. Coal manufactory and burner settings tin can also have substantial effects on slagging.
Excessive coarse coal particles resulting from inadequate pulverizer operation, poor distribution of coal between burners, and poor air distribution tin contribute to slagging. One author's (Hatt'south) feel is that pulverizers that allow a loftier fraction of coarser particles, combined with the presence of pyrite in high-sulfur coal, often results in iron-rich molten ash particles hitting the waterwall. This is especially noticeable in wall-fired boilers. The specifics of the banality design, including the heat release rate and maximum steam temperature also affect the degree of slagging.
An constructive slag control programme should consider all aspects of the boiler, fuel, and combustion arrangement.
Other Corrosion Mitigation Measures
In addition to employing all-time practices for sootblower and pulverizer operation, a variety of other measures may minimize corrosion in circumstances where it becomes a concern.
Flue Gas Oxidation/Reduction Potential. Banality tube wastage has become a more than noticeable problem for many coal-fired units after the installation of LNB OFA systems. These systems purposely create a reducing zone in the hottest part of the boiler to lower NOx production. Normally, the overall air balance is shifted to reduce excess air. The event of these changes may be the creation of zones of reducing temper at diverse elevations in the boiler (which usually occurs during periods of full-load functioning). The elimination of these areas volition greatly reduce the potential for corrosion.
This can be achieved through a careful study of boiler gas catamenia and O2 and CO concentration through a high-velocity thermocouple trace of the boiler. Subsequently this testing, adjustments may exist made such as coal fineness; coal pipe balancing and burner setting for chief airflow and tilt; balance of multiple air inlets, including addition of "boundary air"; and leak reduction.
A related issue, just somewhat separate from the control of furnace temper, is control of temperature. If the coal flame impinges on the banality wall, slag volition be deposited and the very loftier temperatures volition accelerate corrosion. Monitoring of the furnace gas go out temperature equally operating conditions change is important.
Ash Deposit Modification past Chemic Addition. Injection into the banality of chemicals designed to alter the slag chemistry has been used successfully at some plants dealing with loftier-slagging and higher-chlorine coals. An example is the use of Fuel Tech'southward additive to reduce accumulation of hard slag from a high-fe high-sulfur coal. GE reports success with a magnesium and copper additive at a southeastern generating unit of measurement and the Eastern Kentucky Power Cooperative'due south Spurlock Found.
Cladding of Furnace Surfaces. At some plants, corroded tubes have been replaced with more resistant alloys—generally higher chromium (Cr)—or coatings of Cr alloys have been applied. There is a considerable range in the resistance of boiler tube alloys to corrosion, and many boilers may have a mix of tubes and surface treatments applied over time. In the writer's experience, corrosion may continue at the borders of the cladded areas, so "spot" coverage will shortly exist establish to be unsatisfactory. Fully clad boilers are successful at minimizing corrosion when compared to bare wall tubes.
Chlorine and FGD Systems
HCl and other chlorides in flue gas are highly soluble and dissolve and accumulate in the FGD liquor (a slurry with high concentrations of CaCO3 and CaSO4 likewise as Mg and Na). Depending on the materials of construction, the Cl¯ concentrations are controlled to limit corrosion. This variation is important, considering the higher the allowable Cl¯ concentration, the smaller the corporeality of water needed to handle a given increase in chloride loading. This is washed past "blowdown"—release of some wastewater and replacement with freshwater.
Cl¯ is frequently the limiting factor that determines the blowdown charge per unit, and then that over some range, increases in chlorine will probably bear on the blowdown charge per unit. The blowdown water, a wastewater stream, may be handled in various ways. In plants that do non produce a saleable gypsum byproduct from the FGD organization, much (even all) of the wastewater is disposed of along with the FGD solid waste product.
The limits on chloride concentration are determined by the materials of structure of the FGD system, which can range from steel, which can tolerate only relatively low Cl¯ concentrations, to polymer or tile linings that can tolerate concentrations over xxx,000 ppm.
Depending on the details of system design, the blowdown rate will accept to exist increased. This might entail increased pipe and pump sizing. The increased blowdown rate may upshot in increases in costs for additives such as dibasic acid, which will exist lost in the increased blowdown stream. Depending on the disposition of the wastewater, increased wastewater volume volition issue in increased operating costs. In some cases, expansion of the wastewater treatment system will exist required.
Regulatory requirements for FGD wastewater were somewhat uncertain as this article was written in November 2014; futurity changes could have an effect on determining the best options. Some plants must upgrade wastewater treatment systems to reduce discharge of mercury (Hg) or for other reasons, and then increased chlorine may or may not determine the required controls.
Outcome of Chlorine on Mercury Removal. Hg exists in the boiler in both elemental and oxidized (ionic) forms. Substantial evidence exists that HCl in the flue gas reacts with elemental Hg to form Hg ions. These Hg ions are much more soluble than elemental Hg and are captured effectively by wet FGD systems.
At that place is controversy about how much additional Hg may be removed with higher-chlorine coal, merely one of the well-nigh comprehensive studies shows a stiff correlation between the chlorine content of the coal and the amount of Hg removed past the FGD system. The EPA notes: "Operating a wet FGD for SO2 control alongside selective catalytic reduction (SCR) for NOx command with halogen present volition remove more 90% of mercury within the flue gas steam."
Chloride Acid Gas Emissions. The focus of this commodity is on generating units burning high-sulfur coal, nearly all of which have some course of FGD, nearly commonly wet limestone FGD systems. FGD systems remove in excess of 99% of chlorine. Near of these systems will too consequence in SOii emissions of less than 0.2 lb/MMBtu. Units coming together this standard are not required to report emissions of HCl or other acid gasses.
Case Report Summaries
The following case study highlights nowadays examples of the interactions previously discussed and demonstrate the multifariousness of experiences with college-chlorine dress-down. Near of these plants are blending higher-chlorine coal with other coal. At some plants, as indicated, a variety of coal types is used, without whatever systematic blending. Until recently, there were two major producers of higher-chlorine coal, now a third has opened. Prudent purchasing requires a diverse mix of fuels, so buyers have bought a mix of higher- and lower-chlorine coals. Nosotros did non identify a establish that has burned merely college-chlorine coal for a long menses.
Case Study A. This establish has burned a alloy of approximately 40% to lx% higher-chlorine coal with other ILB coal for several years. The units have Babcock & Wilcox wall-fired boilers designed for high-sulfur coal. When a LNB OFA system was installed, the units began to feel serious waterwall corrosion. Coal fineness at this unit of measurement is typically about 60% to seventy% passing 200 mesh, compared to the typical design recommendation of >70% passing 200 mesh.
High temperatures, in the range of 2,500F to two,600F have been observed at the superheater, and corrosion was occurring there. Metallurgical testing showed sulfide corrosion in the furnace. Chloride corrosion was not observed. To control this problem, a chromium alloy blanket has been applied in the furnace. In the operator'south opinion, there is no appreciable chloride corrosion and no deviation in corrosion betwixt higher-sulfur and other ILB coal burned in the units.
The units have an FGD system that is designed to allow circulating water concentrations of chloride ion up to 30,000 ppm, with operation typically at somewhat lower levels. The additional chloride loading from the higher-chlorine coal did non crave modification of the arrangement. In guild to produce gypsum of salable quality, the gypsum is washed to reduce chloride.
Instance Study B. This plant burns a alloy of approximately twenty% to 30% higher-chlorine coal with other ILB coal. The unit is a Combustion Engineering tangentially fired boiler designed for Appalachian coal. In response to corrosion issues encountered after installation of a LNB OFA system, an Inconel overlay has been applied in the boiler. Coal fineness is typically maintained at approximately 75% passing 200 mesh, which finer controls slag formation in the unit. The operators have not observed tube wastage due to called-for the high-chlorine coal.
Some corrosion is beingness seen in the downstream convection passes in the banality. The operators believe that high-sulfur coal and the use of calcium bromide may be contributing to this trouble.
The FGD system is designed for moderate circulating concentrations of chloride. When using higher-chlorine coal, the FGD blowdown rate must exist increased from approximately 100 gpm (using coal with 0.ane% to 0.2% chlorine) to 200–300 gpm while using college-chlorine coal. The operators observe that mercury removal in the FGD system increases with college chlorine content in the coal.
Instance Written report C. This utility burns some college-chlorine coal at several plants. At i of these plants, loftier-chlorine coal is nearly 10% to 20% of the total burned, alternating with other ILB coal, with the coal testing at 0.25% to 0.27% chlorine. Operators have not observed a change in corrosion with the use of college-chlorine coal, including the results of a 90-day test using 90% higher-chlorine coal.
This plant is experiencing waterwall corrosion. Its operators believe this is sulfide corrosion. The LNB OFA system is causing formation of a long coal flame when NOten concentrations are held below 0.two lb NOx/MMBtu. In improver to replacing the LNB with a more conservative pattern, this utility has begun to chrome-coat portions of the boiler.
Superheater slagging is being experienced at this unit of measurement while using some ILB coal that has approximately 2% sodium in ash. This slagging has been controlled past increasing the furnace O2 level, which does increase the NOx loading in the SCR system. Operators observe a strong correlation betwixt slag formation and corrosion and the measured flue gas carbon monoxide.
The FGD arrangement experienced chloride levels outside the initial design allowance while using higher-chloride coal. The blowdown system capacity has been increased and tin can at present maintain chloride concentration of around 3,500 ppm. The scrubbers are beingness upgraded to handle much higher chlorine levels.
Case Study D. This is a cyclone unit that has used from 40% (in a alloy) to 100% college-chlorine coal for extended periods. No boiler corrosion issues accept been encountered.
While using higher-chlorine coal, the FGD system blowdown rate is increased. The byproduct gypsum is rinsed to limit chloride concentration.
Case Written report E. This found has used 100% 0.iii% college-chlorine coal for periods, alternate with other types. The unit of measurement is experiencing serious waterwall slagging, which began with the installation of the LNB OFA system. Operators aspect this partially to the higher chlorine content of the coal only accept not confirmed this with metallurgical tests. The unit, which has no SCR arrangement, is required to operate with a very low NO10 emission rate. NOten command is being provided by a very aggressive staging of combustion with its LNB OFA arrangement.
Example Study F. This establish has used approximately 25% to xxx% higher-chlorine coal in combination with several other types. No corrosion issues are being observed. The plant uses an additive chemical that is intended to reduce acrid gas emissions. Very careful attention is paid to management of mill, burner, air, and sootblower settings.
Lessons Learned
The findings from these case studies and the literature on the corrosion effects of chlorine in coal indicate that college-chlorine coal can exist burned successfully if attention is paid to control of boiler slag and if zones of reducing atmosphere are minimized. In the boiler, at that place does not seem to exist a clear threshold between acceptable and too-high chlorine. A "safety" limit of 0.15% or 0.20% chlorine is not well supported by the prove.
Given that the increasing supply of higher-chlorine coal has encouraged many operators to find ways to use this coal, employ of higher-chlorine coal volition require changes in FGD system h2o direction at about plants. ■
—Rod Hatt (rod_hatt@coalcombustion.com) is president of Coal Combustion Inc. Charles Mann (c.mann@ieplp.com) is managing director of Energy Investors Advisors LLC.
Source: https://www.powermag.com/operational-considerations-when-burning-higher-chlorine-coal/
0 Response to "what to do when coal is fusing in tuiyers"
Postar um comentário